And Ridgeback Biotherapeutics dramatically reduced the number of hospitalizations from COVID-19 in a clinical trial a major finding that could mark a turning point in the coronavirus pandemic. Molnupiravir is an experimental oral antiviral drug developed by Merck and Ridgeback Biotherapeutics.

Buyers Clamor For Merck S Covid 19 Antiviral Molnupiravir But Pricing Is Already Controversial Fiercepharma
Merck revealed the pill molnupiravir cut the risk of COVID.

Merck oral covid drug. On Friday the DOH confirmed 15566 new COVID-19 cases and 199 more deaths. A phase 3 trial of Merck and Ridgeback Biotherapeutics oral antiviral treatment molnupiravir showed it reduced the risk of hospitalization or death by around 50 in Covid patients. Merck announced Wednesday that it has reached an approximately 12 billion deal to supply the US.
Drugmaker Merck Co Inc MRKN has initiated a rolling submission to Health Canada for Molnupiravir an oral antiviral therapy treatment for COVID-19 it said in a statement on Friday. Active cases on the other hand reached 130268. Pharmaceutical company Merck announced on March 6 2021 that its phase 2 clinical trial for an oral medication to fight COVID-19 has promising early findings.
Sept 29 Reuters - Laboratory studies show that Merck Cos experimental oral COVID-19 antiviral drug molnupiravir is likely to be effective against known variants of the coronavirus. At the Interim Analysis 73 Percent of Patients Who Received Molnupiravir Were Hospitalized Through. How Mercks antiviral pill could change the game for COVID-19.
Promising results from clinical trials suggest that the drug may become the first at-home treatment cleared for use by the FDA. Philippines drops to bottom of Bloombergs COVID Resilience Ranking. It is one of several oral pills for COVID-19 being developed and is meant to stop the virus from replicating.
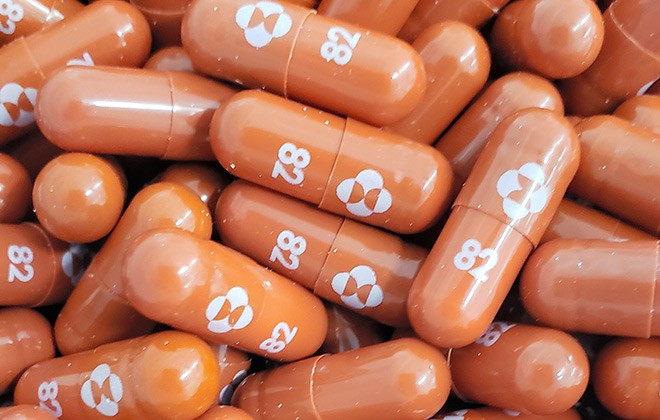
Merck said its new trial will study experimental drug molnupiravir for the prevention of COVID-19 among adults in the same household as someone. If the FDA grants Merck an emergency use authorization for the pill it would be the first oral antiviral for COVID-19. This handout photo obtained May 26 2021 courtesy of Pharmaceutical company Merck shows capsules of.
Merck and Ridgeback say theyll apply as soon as possible to get their COVID. Merck said its new trial will study experimental drug molnupiravir for the prevention of COVID-19 among adults in the same household as someone. The trial will include about 1300 patients who will take.
Merck and partner Ridgeback Biotherapeutics said they plan to seek US. Merck Co is on the brink of plugging a gap in the coronavirus treatment armamentarium after its oral drug molnupiravir cut deaths and hospitalisations in mild or moderate COVID-19 in. WASHINGTON - Pharmaceutical company Merck said Friday it will seek authorisation in the US of its oral drug molnupiravir for COVID-19 after it was shown to reduce the chance newly infected.
Researchers found that the medication called molnupiravir helped reduce the viral load in COVID-19 patients. Mercks Little Brown Pill Could Transform the Fight Against Covid. If cleared Mercks drug would be the first pill shown to treat Covid a potentially major advance in efforts to fight the pandemic.
The antiviral drug molnupiravir still in clinical trials would give doctors an important new. Merck and Ridgeback Biotherapeutics have announced that investigational oral antiviral drug molnupiravir MK-4482 EIDD-2801 has been shown to markedly reduce the risk of hospitalization or death in at-risk non-hospitalized adults with mild-to-moderate COVID-19. Merck and partner company Ridgeback Biotherapeutics announced the results.
According to the two companies an interim analysis of its Phase III MOVe-OUT trial indicated that the drug molnupiravir. The results were so convincing that outside trial monitors directed Merck. Pharmaceutical company Merck has said it will seek authorisation in the US for an oral drug for Covid-19 after the pill showed compelling results.
An antiviral pill being developed by Merck Co. Reuters -Laboratory studies show that Merck Cos experimental oral COVID-19 antiviral drug molnupiravir is likely to be effective against known variants of the coronavirus. Merck to seek US nod for oral COVID-19 drug.
Pfizer and Merck Co. Merck to seek authorisation for oral Covid drug. If it gets authorization molnupiravir which is designed to introduce errors into the genetic code of the virus would be the first oral antiviral medication for COVID-19.
Merck and Ridgebacks Investigational Oral Antiviral Molnupiravir Reduced the Risk of Hospitalization or Death by Approximately 50 Percent Compared to Placebo for Patients with Mild or Moderate COVID-19 in Positive Interim Analysis of Phase 3 Study. October 1 2021 600 am ET. Infectious Disease COVID-19 Oral COVID Drug Slashes Hospitalization and Death Risk Merck Says However expert notes drug resistance inducing viral mutations may be a problem.
Government 17 million doses of molnupiravir an oral antiviral treatment for. Each launched pivotal clinical studies of new experimental oral COVID-19 drugs building on previous work to provide outpatient alternatives for patients. The Philippines is battling.
Treatment with the drug called molnupiravir lowered the risk of hospitalization or death by roughly 50 in study participants who all had mild or moderate COVID-19. Merck says its antiviral pill reduces COVID-19 hospitalizations. Merck is studying whether its medicine molnupiravir can prevent COVID-19 in adults who live with a symptomatic patient with a confirmed coronavirus infection.
Merck to seek authorisation for oral Covid-19 drug. By Deena Beasley. Emergency use authorization for the pill as soon as possible and to make regulatory applications worldwide.
Japan Seeking Supply Of Merck S Oral Covid 19 Drug This Year The Asahi Shimbun Breaking News Japan News And Analysis

Japan Seeking Supply Of Merck S Oral Covid 19 Drug This Year The Asahi Shimbun Breaking News Japan News And Analysis

Covid Live Updates Asia Pacific Nations Seek Early Supply Of Merck S Home Covid Pill The New York Times

Covid Molnupiravir Drugmaker Merck Seeks Marketing Approval Sortiraparis Com

Merck Ridgeback S Oral Drug Lowers Hospitalisation Risk In Covid 19 Trial

Pfizer Merck Launch New Trials Of Oral Covid 19 Drugs Reform Austin

Japan Seeking Supply Of Merck S Oral Covid 19 Drug This Year The Asahi Shimbun Breaking News Japan News And Analysis

Merck Mrk Molnupiravir Pill Could Change The Fight Against Covid Bloomberg
YOU MAY LIKE :









/cloudfront-us-east-1.images.arcpublishing.com/dmn/WYHIJMT4AK4Z75FFTJRTAYLE24.jpg)

